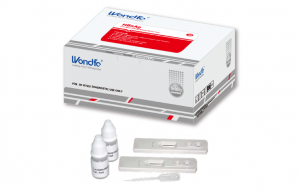
Description
| Product Code: |
W290
|
| Detection: | Spike receptor-binding domain (RBD) antibodies of 2019-nCoV |
| Specimen: | Human fingerstick whole blood, venipuncture whole blood, serum or plasma specimen |
| Kit Content: |
|
| Test per kit: | 5 or 10 or 20 or 25 or 40 |
Fast
Test result in 15 minutes. Simple to perform and user friendly.
Flexible
Qualitative & semi-quantitative detection of spike receptor-binding domain (RBD) antibodies of SARS-CoV-2.
Convenient
24 months shelf life at room temperature.
Highly Informative
High accuracy level. Versatile Sample Type.